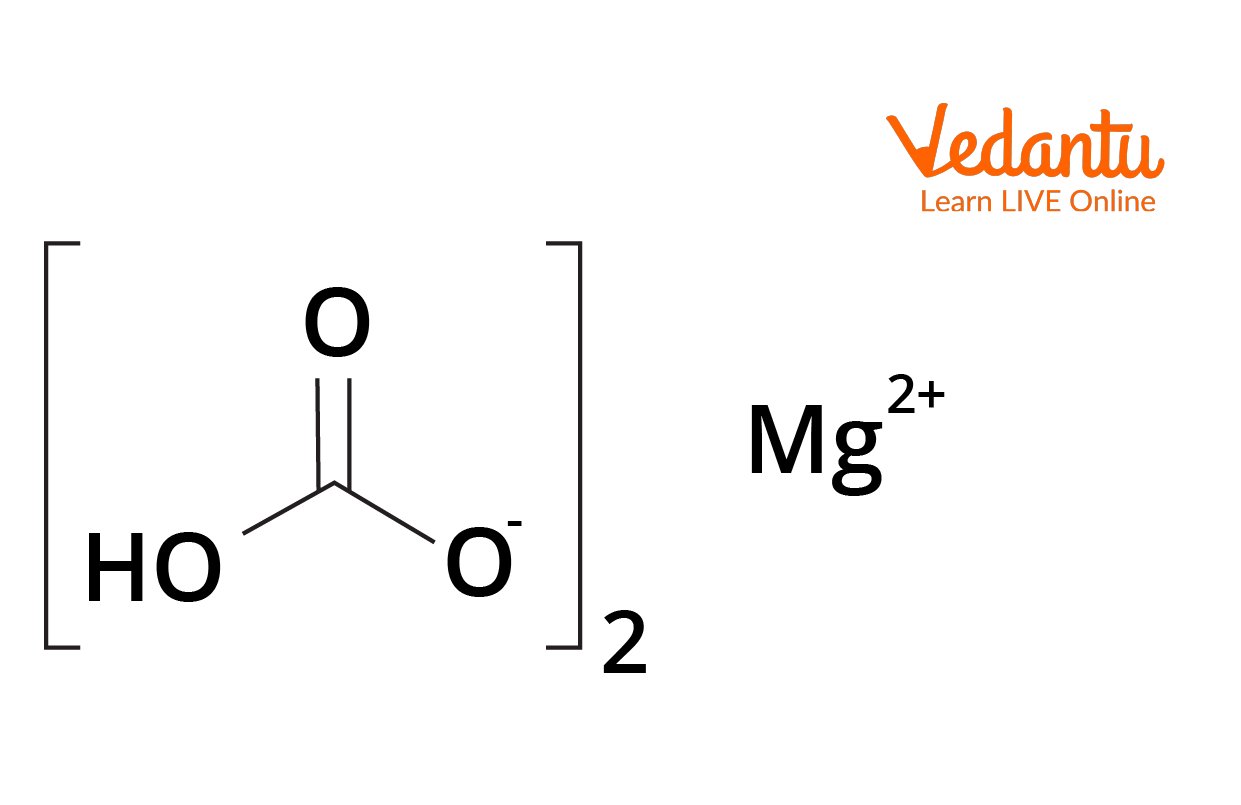
Magnesium Bicarbonate Decomposition Temperature . The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate, carbon dioxide, and. One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. In the present study, we researched the specific composition of samples prepared at different decomposition temperatures of magnesium bicarbonate. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little mgo, mg (oh)2, trace. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding.
from www.vedantu.com
The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little mgo, mg (oh)2, trace. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate, carbon dioxide, and. In the present study, we researched the specific composition of samples prepared at different decomposition temperatures of magnesium bicarbonate. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric.
Magnesium Bicarbonate Learn Important Terms and Concepts
Magnesium Bicarbonate Decomposition Temperature One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little mgo, mg (oh)2, trace. In the present study, we researched the specific composition of samples prepared at different decomposition temperatures of magnesium bicarbonate. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate, carbon dioxide, and. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric.
From www.degruyter.com
Synthesis of magnesium carbonate hydrate from natural talc Magnesium Bicarbonate Decomposition Temperature The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate,. Magnesium Bicarbonate Decomposition Temperature.
From keystagewiki.com
Thermal Key Stage Wiki Magnesium Bicarbonate Decomposition Temperature Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. In the present study, we researched the specific composition of samples prepared at. Magnesium Bicarbonate Decomposition Temperature.
From www.chemistrylearner.com
Magnesium Bicarbonate Facts, Formula, Synthesis, Properties, Uses Magnesium Bicarbonate Decomposition Temperature Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate, carbon dioxide, and. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. One of the main steps that requires a lot of energy are magnesium bicarbonate thermal. Magnesium Bicarbonate Decomposition Temperature.
From bmp-brah.blogspot.com
Mgco3 Balanced Equation bmpbrah Magnesium Bicarbonate Decomposition Temperature Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. In the present study, we researched the specific composition of samples prepared at different decomposition temperatures of magnesium bicarbonate. One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate, carbon. Magnesium Bicarbonate Decomposition Temperature.
From www.youtube.com
of Sodium Bicarbonate (Baking Soda) YouTube Magnesium Bicarbonate Decomposition Temperature In the present study, we researched the specific composition of samples prepared at different decomposition temperatures of magnesium bicarbonate. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and. Magnesium Bicarbonate Decomposition Temperature.
From brainly.in
formula of magnesium bicarbonate Brainly.in Magnesium Bicarbonate Decomposition Temperature In the present study, we researched the specific composition of samples prepared at different decomposition temperatures of magnesium bicarbonate. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate, carbon dioxide, and. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a. Magnesium Bicarbonate Decomposition Temperature.
From www.chegg.com
Solved Consider the of magnesium carbonate Magnesium Bicarbonate Decomposition Temperature Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little mgo, mg (oh)2, trace. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium. Magnesium Bicarbonate Decomposition Temperature.
From www.youtube.com
20 g of magnesium carbonate sample on heating to give carbon Magnesium Bicarbonate Decomposition Temperature One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate, carbon dioxide, and. The magnesium bicarbonate solution was obtained by carbonization of light. Magnesium Bicarbonate Decomposition Temperature.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Magnesium Bicarbonate Decomposition Temperature The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little. Magnesium Bicarbonate Decomposition Temperature.
From www.numerade.com
SOLVED thermal of magnesium nitrate and magnesium hydroxide Magnesium Bicarbonate Decomposition Temperature One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. Drying the resulting solution causes the magnesium bicarbonate. Magnesium Bicarbonate Decomposition Temperature.
From www.researchgate.net
(PDF) An Experimental Study of the and Carbonation of Magnesium Bicarbonate Decomposition Temperature In the present study, we researched the specific composition of samples prepared at different decomposition temperatures of magnesium bicarbonate. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. The decomposition products obtained at. Magnesium Bicarbonate Decomposition Temperature.
From www.scientific.net
Effect of Temperature on of Magnesium Bicarbonate Magnesium Bicarbonate Decomposition Temperature The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little mgo, mg (oh)2, trace. Drying the resulting solution causes the magnesium. Magnesium Bicarbonate Decomposition Temperature.
From www.chegg.com
Solved of magnesium carbonate produces Magnesium Bicarbonate Decomposition Temperature One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little mgo, mg (oh)2, trace. Drying the resulting solution causes the magnesium. Magnesium Bicarbonate Decomposition Temperature.
From pubs.rsc.org
Probing the thermal stability and the mechanism of a Magnesium Bicarbonate Decomposition Temperature The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little mgo, mg (oh)2, trace. Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. In the present study, we researched the specific composition of samples prepared. Magnesium Bicarbonate Decomposition Temperature.
From www.youtube.com
How to Balance MgCO3 = MgO + CO2 of Magnesium carbonate Magnesium Bicarbonate Decomposition Temperature Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding magnesium carbonate, carbon dioxide, and. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. In the present study, we researched the specific composition of samples prepared at different decomposition temperatures of magnesium bicarbonate. Drying the resulting solution causes the magnesium. Magnesium Bicarbonate Decomposition Temperature.
From byjus.com
20 gof magnesium carbonate sample on heating to give carbon Magnesium Bicarbonate Decomposition Temperature Drying the resulting solution causes the magnesium bicarbonate to decompose, yielding. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little mgo, mg (oh)2, trace. The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. One of the main steps that requires a lot of energy are. Magnesium Bicarbonate Decomposition Temperature.
From edu.rsc.org
Thermal of metal carbonates Experiment RSC Education Magnesium Bicarbonate Decomposition Temperature In the present study, we researched the specific composition of samples prepared at different decomposition temperatures of magnesium bicarbonate. One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little mgo, mg (oh)2, trace. The magnesium bicarbonate solution was obtained. Magnesium Bicarbonate Decomposition Temperature.
From www.markedbyteachers.com
The of Sodium Hydrogen Carbonate GCSE Science Marked Magnesium Bicarbonate Decomposition Temperature The magnesium bicarbonate solution was obtained by carbonization of light calcined magnesia with co 2 gas at atmospheric. One of the main steps that requires a lot of energy are magnesium bicarbonate thermal decomposition. The decomposition products obtained at 90 °c is 4mgco3mg (oh)24h2o based and contain a little mgo, mg (oh)2, trace. Drying the resulting solution causes the magnesium. Magnesium Bicarbonate Decomposition Temperature.